Technologies
NextGen, GenX, and Opt AAV3 Vectors
Recombinant AAV vectors have been, or are currently being, used in over 300 Phase I/II/III trial in humans and have shown clinical efficacy in a wide variety of human diseases. AAV vectors have already been approved in Europe and the United States. Despite these remarkable achievements, the full potential of AAV vectors, when composed of the naturally occurring, wild-type (WT) capsids and genomes, is unlikely to be realized for the following reasons:
- AAV capsid is targeted for degradation by the host cell enzymes, which leads to host immune responses to capsid proteins
- The single-stranded AAV genome is transcriptionally-inactive, which impacts on the expression levels of transgenes
…sAAVient has overcome both limitations.
sAAVient Therapeutics has developed NextGen AAV3 vectors, illustrated here, which decrease the natural propensity of the host immune response.
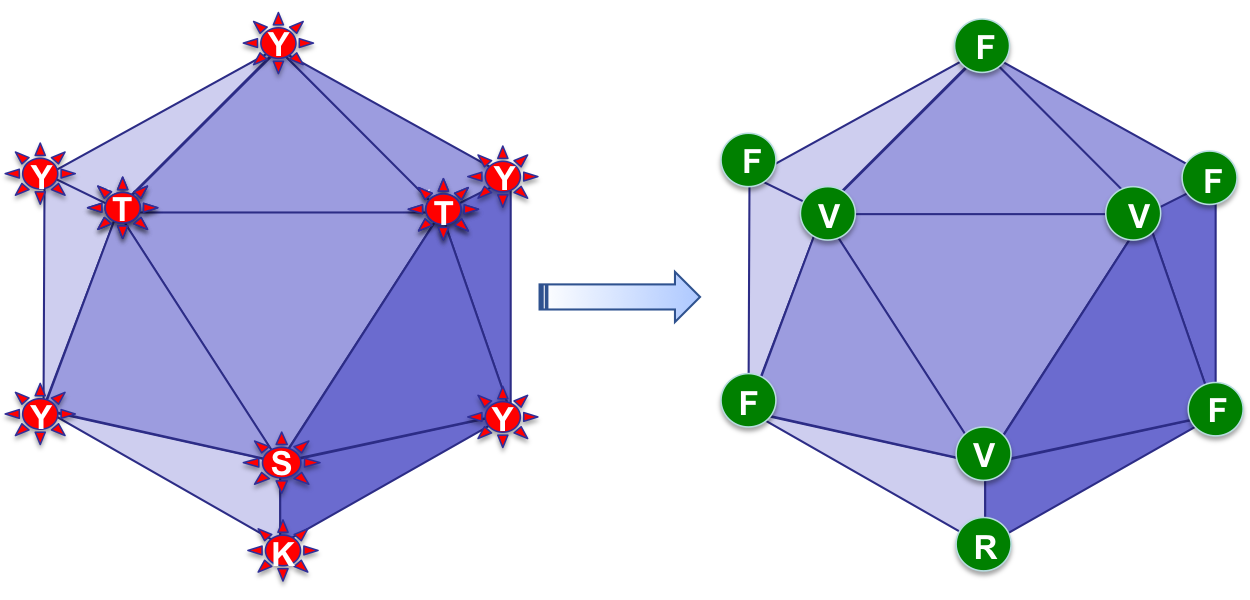
Surface-exposed tyrosine (Y), serine (S), threonine (T), and lysine (K) residues in the first generation AAV3 vectors are mutagenized to phenylalanine (F), valine (V), and arginine (R) residues, respectively, in the NextGen AAV3 vectors that are more efficient and less immunogenic.
sAAVient Therapeutics has overcome the limitation of the wild-type single-stranded AAV genome with the development of GenX AAV3 vectors, depicted below.
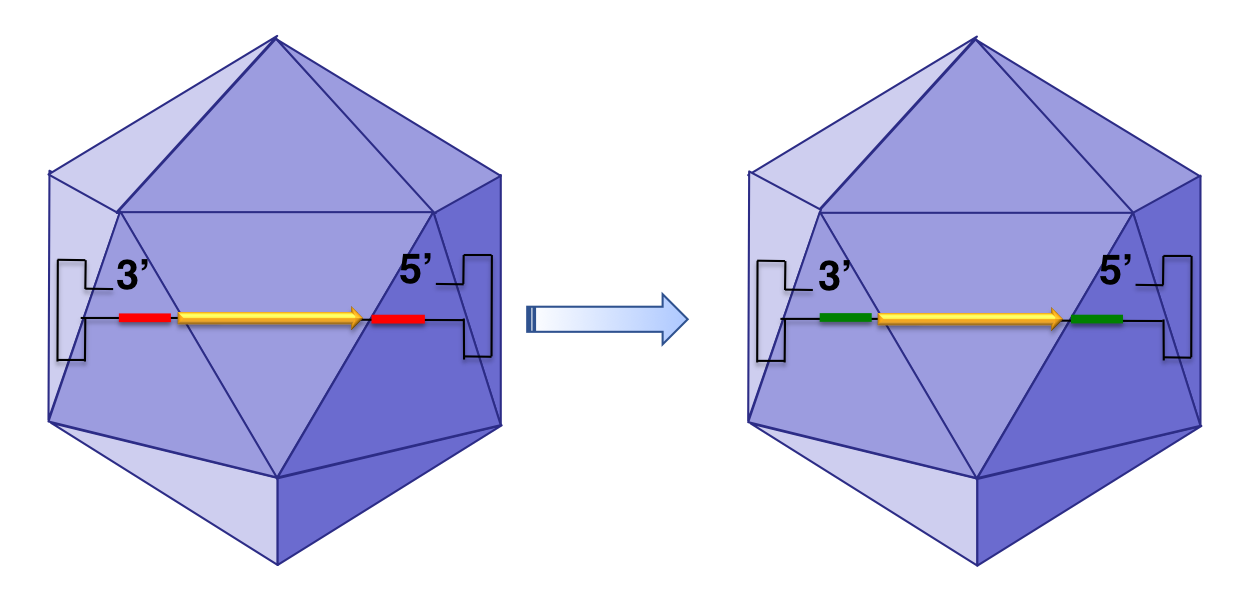
Specific DNA sequences in the inverted terminal repeats in the ssAAV genome (depicted in red) are replaced by a substitute sequence (depicted in green) in the GenX AAV vectors that mediate more efficient transgene expression.
sAAVient Therapeutics has also demonstrated that combining NextGen capsids with GenX genomes leads to the generation of optimized (Opt) AAV vectors, shown schematically below, that are more efficient at further reduced doses.
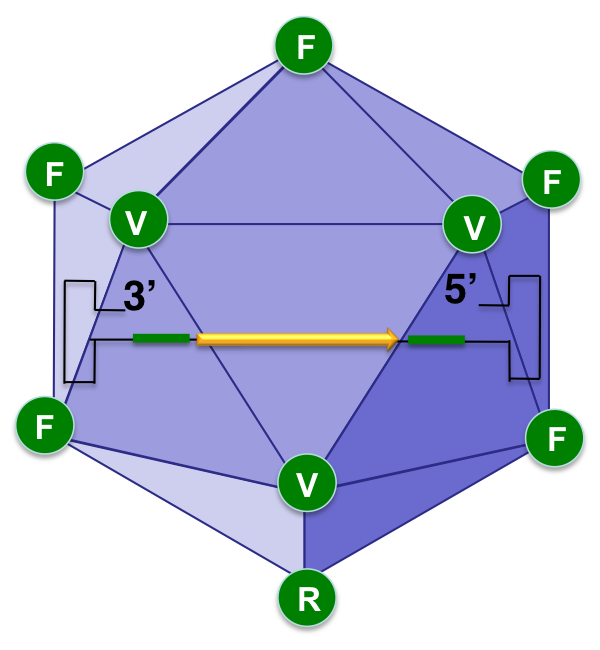
Encapsidation of the GenX genome into the NextGen capsid leads to the generation of more efficient optimized (Opt) AAV vectors at further reduced doses.
sAAVient Therapeutics’s NextGen, GenX, and Opt AAV3 vectors have been shown to be highly efficient in human cells in vitro, and in mouse models in vivo. sAAVient’s NextGen AAV3 vectors have also been evaluated for their safety and efficacy in “humanized” mice and in non-human primates, and shown to perform significantly better than first generation AAV5 and AAV8 vectors, the two other serotype vectors currently being used by other AAV hemophilia gene therapy companies.