Our Science
Development of NextGen and Optimized AAV vectors
Several first generation of AAV serotype vectors (AAV2, AAV5, AAV6, AAV8, AAVrh10, and AAVrh74) vectors, composed of naturally occurring capsids, that have been used by other AAV hemophilia gene therapy companies, but none of these serotype vectors are truly human liver-tropic. Furthermore, they have been shown to induce immune responses, especially at high doses since the host immune system cannot distinguish between AAV as a virus versus AAV as a vector, and since the wild-type AAV did not evolve for the purposes of delivery of therapeutic genes.
sAAVient Therapeutics has generated proprietary capsid-modified next generation (NextGen), genome-modified generation X (GenX), and the combination of the two, optimized (Opt) adeno-associated virus serotype 3 (AAV3) vectors. The remarkable specificity and efficacy of AAV3 vectors for human liver was first documented by sAAVient’s scientists nearly two decades ago. Subsequently, sAAVient’s NextGen, GenX, and Opt AAV3 vectors were shown to be highly efficient in human cells in vitro, and in mouse models in vivo. sAAVient’s NextGen AAV3 vectors have also been evaluated for their safety and efficacy in “humanized” mice and in non-human primates.
sAAVient Therapeutics utilizes the natural tropism of AAV3 for human hepatocytes, and in pre-clinical studies, NextGen AAV3 vectors have been shown to perform significantly better than first generation AAV2, AAV5, AAV6 and AAV8, and AAVrh74 vectors, and express therapeutic levels of human clotting factor IX (hFIX) in “humanized” mice and in non-human primates at significantly lower vector doses.
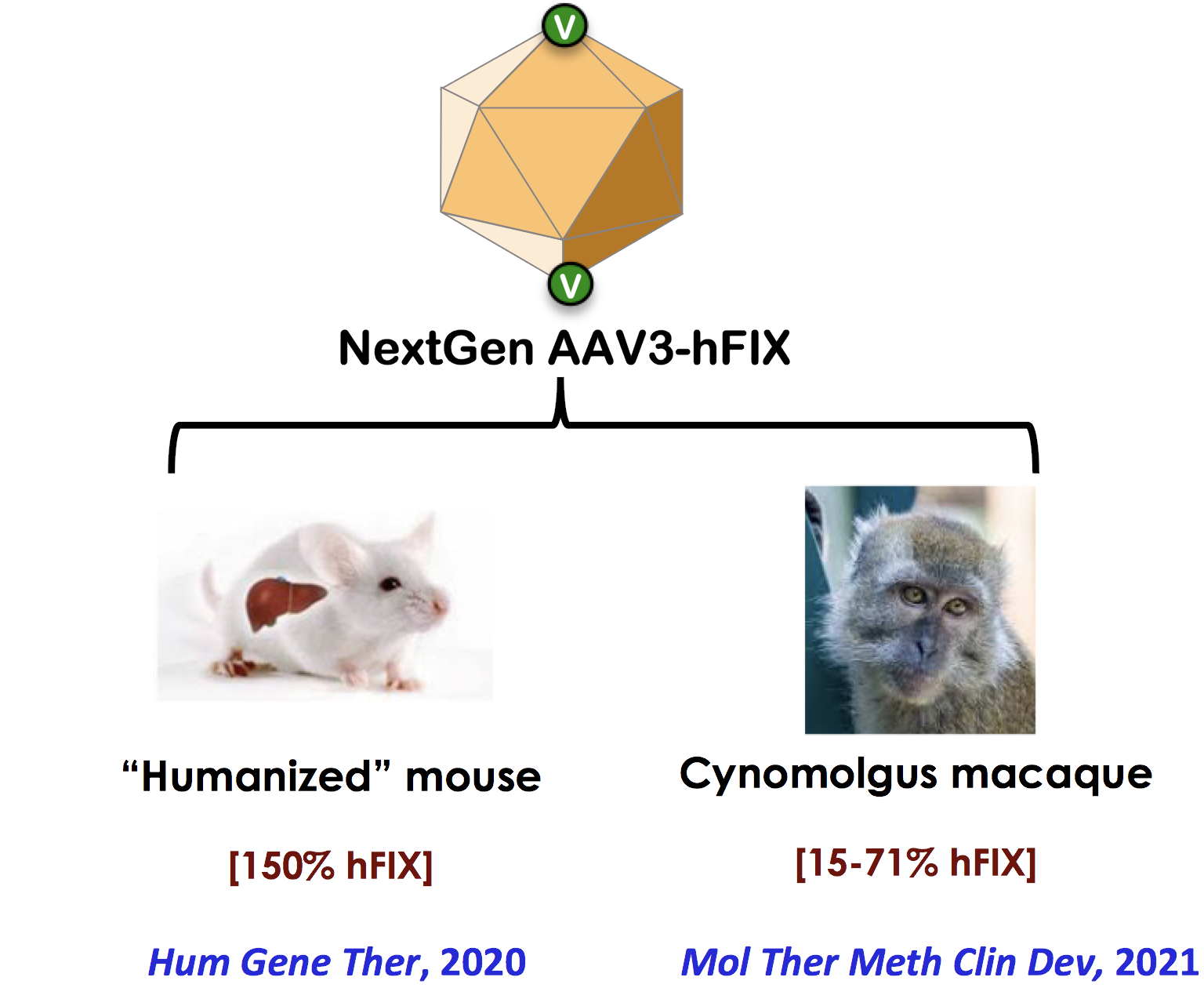
Thus, in contrast to the high vector doses that are currently being used with other serotypes, that require prophylactic immune-suppression, optimized AAV3 serotype vectors also offer the potential advantages of being less immunogenic, and more cost-effective, for their use in a wide variety of human liver diseases in general, and hemophilia B in particular, ensuring translation to the clinic with higher probability of success.
sAAVient Therapeutics has also demonstrated that AAV3 vectors are significantly more efficient than AAV5, AAV6, and AAV8 vectors in transducing primary human liver sinusoidal endothelial cells (LSECs), where clotting factor VIII (hFVIII) is expressed, and secreted from. In contrast, in four different human clinical trials, primary human hepatocytes.
With the combined extensive experience of the scientific founders in AAV biology and vector development spanning more than four decades, sAAVient Therapeutics is ideally positioned to overcome the technological challenges that a number of other hemophilia gene therapy companies using less efficient, first generation of AAV serotype vectors have encountered and continue to encounter. The sAAVient Therapeutics team possesses novel insights into the basic biology of AAV vectors in general, and AAV3 vectors in particular, that promise to lead to a permanent cure of both hemophilia B and hemophilia A with a one-time treatment.